Niemann-Pick Disease Type C Market Size, Growth Rate & Companies 2025-2033 | DataM Intelligence
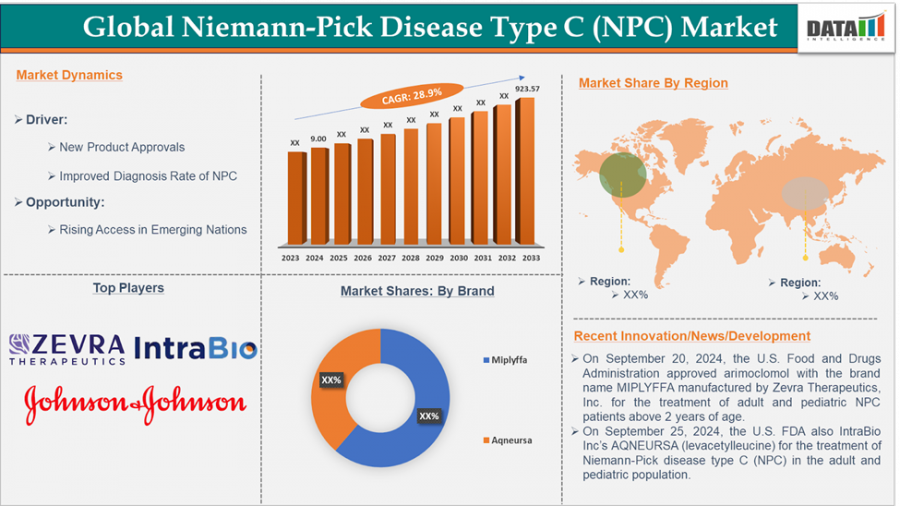
The Global Niemann-Pick Disease Type C Market is projected to grow from $9M in 2024 to $923.57M by 2033, at a strong CAGR of 28.9% driven by rare disease Focus
AUSTIN, TX, UNITED STATES, May 22, 2025 /EINPresswire.com/ -- Niemann-Pick Disease Type C (NPC) Market – 2025 Outlook
Niemann-Pick Disease Type C (NPC) is a rare, inherited condition that affects the body’s ability to transport cholesterol and other lipids within the cells.
The Niemann-Pick Disease Type C (NPC) Market Size reached US$ 9.00 million in 2024 and is expected to reach US$ 923.57 million by 2033, growing at a CAGR of 28.9% during the forecast period of 2025-2033.
When this function is impaired, lipids accumulate in the liver, spleen, and brain, eventually leading to neurodegenerative decline. Though rare, the impact of NPC is profound, often affecting children and progressively worsening over time. However, the NPC market is experiencing a wave of developments that are bringing hope to families and healthcare professionals alike.
Market Dynamics and Growth Forecast
The NPC market is relatively small due to the rarity of the disease, but it is growing at an impressive rate. With increasing awareness, more patients are being diagnosed earlier, and researchers are making progress in treatment development. This is triggering demand for new diagnostic tools, therapies, and patient support services.
In recent years, a handful of treatments have either been approved or advanced in clinical trials, setting the stage for significant market expansion. The anticipated compound annual growth rate (CAGR) is high, driven largely by advancements in precision medicine and increasing focus on rare diseases. Innovative therapies are moving from experimental to accessible, giving the market both value and purpose.
To Download Sample Report: https://www.datamintelligence.com/download-sample/niemann-pick-disease-type-c-market
Regional Outlook
North America remains the leader in NPC market share, with the United States at the forefront. This is due to better healthcare infrastructure, early adoption of orphan drug incentives, and strong involvement of biotech firms focused on rare conditions.
Europe is also showing steady development, especially in countries like Germany, the UK, and France, where early detection programs and rare disease networks are gaining traction.
Asia-Pacific, particularly Japan, is beginning to emerge as a significant region in the NPC space. Diagnostic efforts are increasing, and pharmaceutical companies in the region are getting involved in research collaborations.
Key Market Players
A small but dedicated group of companies is working on breakthrough therapies for NPC
Zevra Therapeutics.
IntraBio.
Johnson & Johnson Services, Inc.
Emerging Market Players
Cyclo Therapeutics, Inc
Azafaros B.V.
Market Segmentation:
By Drug: Arimoclomol (MIPLYFFA), Levacetylleucine (AQNEURSA), Miglustat
By Patient Type: Adult, Pediatric
By Region Divided on: North America, Europe, South America, Asia Pacific, Middle East, and Africa
Latest News – USA
In the U.S., the years 2024 and early 2025 have marked significant milestones in the advancement of NPC therapy. For the first time, meaningful options have entered the market that offer more than symptom control. Families and physicians now have access to therapies that can potentially alter the course of the disease.
There is also a growing movement to support early diagnosis, including newborn screening initiatives and genetic counseling. As the conversation around rare diseases becomes louder, NPC is gaining visibility within federal health policy and funding initiatives.
Additionally, biotech firms based in the U.S. are continuing to invest in clinical trials, working closely with the FDA to fast-track review processes for rare disease treatments. The future looks more hopeful now than ever before.
Latest News – Japan
In Japan, awareness of NPC is slowly but steadily increasing. The country has seen a rise in diagnosed pediatric cases in recent years, which is prompting both government and private health organizations to strengthen early detection strategies.
Hospitals and research centers are collaborating to gather data on NPC to help guide treatment protocols. Although treatment options remain limited, Japanese researchers are actively participating in global studies to bring promising therapies to local patients.
Japan’s approach to rare diseases often involves a patient-first philosophy, and this can be seen in the level of care provided to NPC patients. Government programs are also beginning to consider NPC in their rare disease support initiatives, a step that could improve access to future treatments.
Challenges and Opportunities
The biggest challenge in the NPC market remains its rarity. With limited patient numbers, it's difficult to run large-scale clinical trials or attract widespread commercial investment. However, this has also led to a close-knit community of researchers, healthcare providers, and patient advocacy groups who are deeply committed to finding solutions.
Potential exists in genetic therapies, tailored medical treatments, and international research partnerships. With regulatory frameworks becoming more supportive of rare disease treatments, the door is open for smaller firms to bring forward bold, innovative ideas.
Conclusion
The NPC market, while small in size, is large in determination. From new drug approvals to international research partnerships, momentum is building. As diagnostic technology improves and awareness increases, more individuals will receive a timely diagnosis opening the door to earlier and more effective treatment.
Families living with NPC now have reasons to be hopeful. With science advancing and society paying closer attention to rare diseases, the future of NPC care is no longer uncertain it’s actively being shaped by passionate teams around the world.
Explore Recent Trending Reports By DataM Intelligence
Genetic Testing Market Size, Share In 2025
Dysphagia Management Market Size, Share
Stay informed with the latest industry insights - start your subscription now: https://www.datamintelligence.com/reports-subscription
Sai Kiran
DataM Intelligence 4Market Research
+1 877-441-4866
Sai.k@datamintelligence.com
Visit us on social media:
LinkedIn
X
Distribution channels: Healthcare & Pharmaceuticals Industry
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release