Pompe Disease Market Size in the 7MM to Reach USD 2,240.4 Million by 2035 - Epidemiology Report by IMARC Group
The report also provides a detailed analysis of the current Pompe disease marketed drugs and late-stage pipeline drugs.
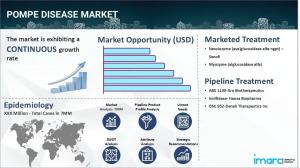
BROOKLYN, NY, UNITED STATES, July 2, 2025 /EINPresswire.com/ -- The Pompe disease market size reached a value of USD 1,336.1 Million in 2024. Looking forward, IMARC Group expects the 7MM to reach USD 2,240.4 Million by 2035, exhibiting a growth rate (CAGR) of 4.81% during 2025-2035.The Pompe disease market is centered around the diagnosis and treatment of a rare genetic disorder known as glycogen storage disease type II. The disorder results in toxic accumulation of glycogen in organs and muscles as a result of acid alpha-glucosidase (GAA) enzyme deficiency. The market comprises enzyme replacement therapies (ERT), novel gene therapies, supportive care, diagnostic screening, and patient services.
Market Growth Outlook
Enzyme replacement therapy is still the mainstay of management. Standard formulations Myozyme and Lumizyme continue to manage infantile- and late-onset presentations, but second-generation agents such as Nexviazyme (avalglucosidase alfa) and Pombiliti (cipaglucosidase alfa) provide enhanced efficacy, greater tissue targeting, and diminished immune response. Combination regimes pairing ERT with stabilizers—like cipaglucosidase and miglustat—are providing better results in late-onset patients.
Gene therapies and substrate reduction strategies are on the move. Pipeline products from such firms as Amicus and Sanofi are moving through early studies. Incentives from regulators—such as orphan designations and fast-track routes—are moving them forward quickly. Diagnostics and screening are growing as well. With increasing newborn and genetic screening programs around the world, increasing numbers of cases are detected early, allowing for timely treatment initiation. Telemedicine and multidisciplinary care teams are arising to assist patients and families outside of infusion centers.
Request to get a PDF Sample Report: https://www.imarcgroup.com/pompe-disease-market/requestsample
The report also provides a detailed analysis of the current Pompe disease marketed drugs and late-stage pipeline drugs.
In-Market Drugs
Drug Overview
Mechanism of Action
Regulatory Status
Clinical Trial Results
Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
Drug Overview
Mechanism of Action
Regulatory Status
Clinical Trial Results
Drug Uptake and Market Performance
Competitive Landscape
The competitive landscape of the Pompe disease market has been studied in the report with the detailed profiles of the key players operating in the market.
Sanofi
Aro Biotherapeutics
Hansa Biopharma
Denali Therapeutics Inc
Buy the full Pompe Disease Market Epidemiology Report: https://www.imarcgroup.com/checkout?id=29175&method=809
7 Major Countries Covered
United States
Germany
France
United Kingdom
Italy
Spain
Japan
IMARC Group Offers Other Reports:
Dyssomnias Market Report, Epidemiology Industry Trends, Share, Size, Growth, Opportunity, and Forecast
Minimal Residual Disease Market Report: Epidemiology, Industry Trends, Share, Size, Growth, Opportunity, and Forecast
Elena Anderson
IMARC Services Private Limited
+1 631-791-1145
email us here
Distribution channels: Business & Economy, Companies, Healthcare & Pharmaceuticals Industry
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release